Understanding Water pH Levels
Water pH level refers to the measure of acidity or alkalinity of water and is an important factor to consider when preparing for herbicide applications. The pH scale ranges from zero to 14, with seven being neutral. A pH value below seven indicates acidity, while a value above seven indicates alkalinity. Different herbicides have specific pH requirements for optimal performance. When the water pH deviates from the recommended range, it can affect the stability, solubility, and activity of herbicides. Furthermore, water pH can influence the availability of herbicide-active ingredients and their ability to bind to target plant surfaces. This interaction between water pH and herbicide efficacy highlights the importance of maintaining the appropriate pH level for successful weed control.
Effects of pH on Flumioxazin
Flumioxazin was the first active ingredient that Alligare brought to market back in 2002 to control the noxious and invasive species, Kochia. This chemistry still plays an important role in our industry and the markets we serve. It is the stand-alone A.I. in our Flumigard products and expands the weed spectrum and resistance-fighting qualities of our two newest bare-ground products, Ballast and Mainline. With all herbicides, it is important to follow best management practices during mixing operations to be sure the products being applied are mixed into a solution that will maintain the stability of the herbicide(s) and maximize efficacy and the longevity of control provided.
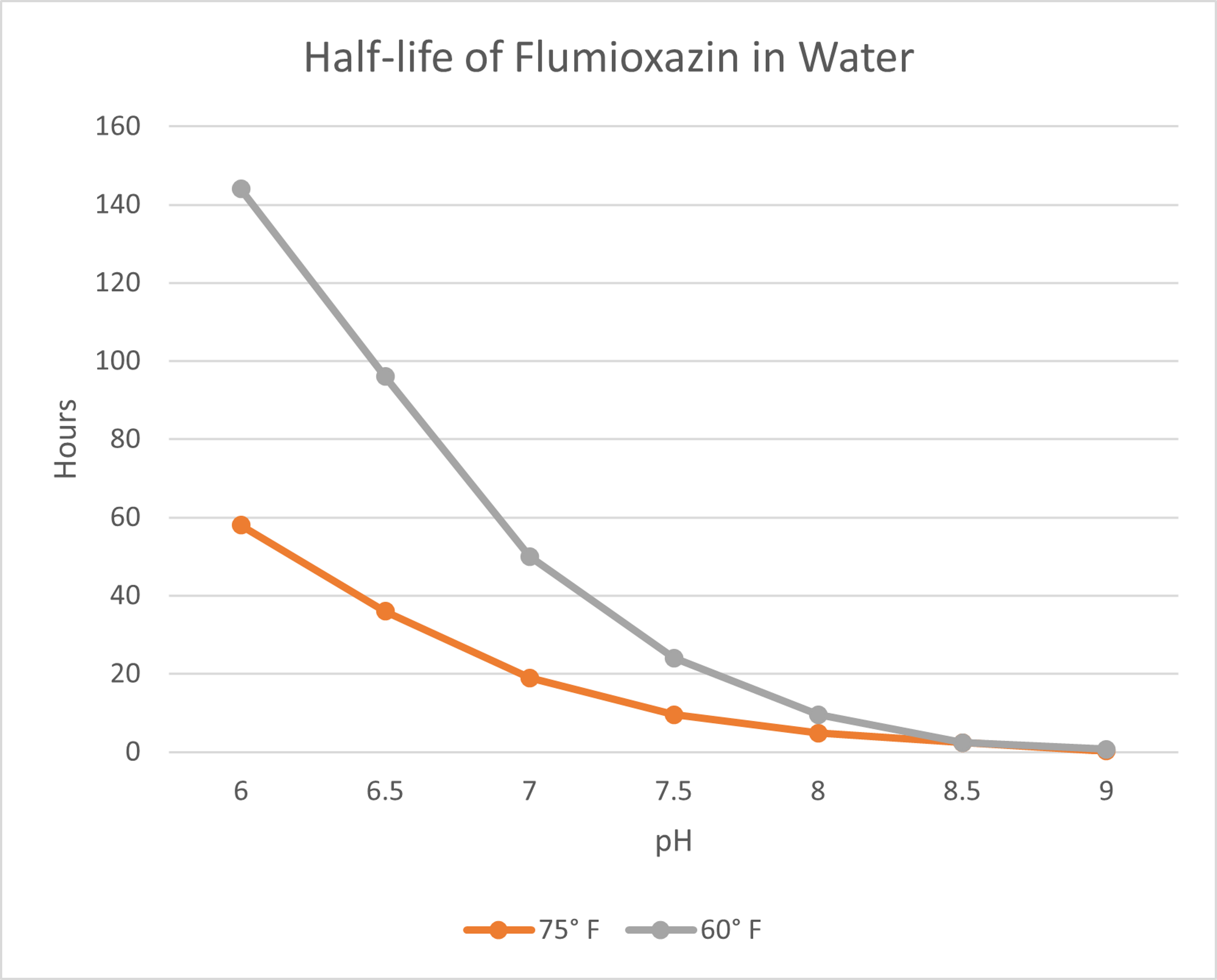
All herbicides degrade once they are introduced to the environment. Photolysis is the breakdown of a chemical when introduced to light. Biological degradation is attributed to microorganisms such as fungi and bacteria breaking down the chemistry in the soil. For flumioxazin, the primary mechanism for degradation is hydrolysis, which is the breakdown of the herbicide in water. Water pH and temperature both play a role in how quickly this degradation will happen and knowing this allows an applicator to take mitigating steps to slow the degradation process. A half-life is the time it takes for a certain amount of a herbicide to be reduced by half, once introduced to the environment. As you can see by the chart below, the higher the water temperature, and more importantly, the higher the pH, the shorter the half-life of the chemistry. Simply put, as pH approaches nine, the availability of the product in the spray tank is reduced to hours or mere minutes.
The Importance of Adjuvants
As previously mentioned, different herbicides will have varying pH specifications depending on the active ingredient(s) or sometimes chemical family. For instance, sulfonylurea herbicides prefer an alkaline environment. The increased tank pH makes this family of herbicides more soluble in water, resulting in increased chemical activity and greater efficacy. Most herbicides, however, like Flumioxazin, prefer a more acidic tank-mix environment. In both instances, there are buffering adjuvants available that can either raise or lower the tank mix pH to accommodate the specific herbicide's label recommendations. The water source being used by the herbicide applicator will rarely be at a pH of seven, so it is always important to test your tank pH prior to mixing. Once the source pH is known, the appropriate adjuvant should be added to the spray tank, and allowed to disperse, before adding any herbicides. This small step will help optimize the effectiveness and overall efficacy of the spray application.
A Case Study
There are certain herbicides, like Glyphosate, that when used as a tank-mix partner, can effectively alter the pH of a spray solution. Dr. Scott Nissen from Colorado State University conducted a series of experiments utilizing Alligare's Dryphosate 75SG (Glyphosate) and Mainline (Flumioxazin + Imazapic) to test if the acidic properties of glyphosate would lower the pH of a spray solution to the point that the flumioxazin component of the Mainline herbicide would remain stable. In these experiments, Dr. Nissen used unbuffered tap water with a pH of 7.7. After adding the Mainline to the tap water, the pH of the solution rose to 8.4, which would reduce the availability of the flumioxazin element of the herbicide. In the second experiment, Dr. Nissen added Dryphosate to tap water from the same source. The solution quickly became acidic, as the pH dropped to 4.0. Finally, Mainline herbicide was added into the now acidic solution, resulting in a final pH of 4.2, thus stabilizing the Flumioxazin element of the mix. This can also be accomplished through the use of an acidifier adjuvant. In both instances, the Glyphosate or acidifier should be added to the spray tank prior to the addition of any other herbicides.

Conclusion
It is important that vegetation managers regularly test the pH level of their water sources and make the necessary adjustments. Always consult the herbicide labels and product information to ensure compatibility and recommended water pH. Proper water management practices, such as pH buffering and treatment, can help maintain the desired pH range for optimal herbicide efficacy. By taking this into consideration, vegetation managers can enhance the effectiveness of herbicides and improve weed control for their customers and the industries they serve.
